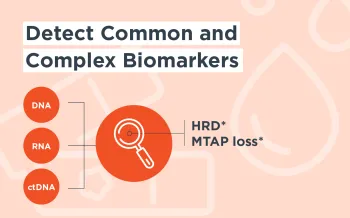
FoundationOne®CDx and FoundationOne®Liquid CDx are qualitative next-generation sequencing based in vitro diagnostic tests for advanced cancer patients with solid tumors and are for prescription use only. FoundationOne CDx utilizes FFPE tissue and analyzes 324 genes as well as genomic signatures. FoundationOne Liquid CDx analyzes 324 genes utilizing circulating cell-free DNA and is FDA-approved to report short variants in 311 genes. The tests are companion diagnostics to identify patients who may benefit from treatment with specific therapies in accordance with the therapeutic product labeling. Additional genomic findings may be reported and are not prescriptive or conclusive for labeled use of any specific therapeutic product. Use of the tests does not guarantee a patient will be matched to a treatment. A negative result does not rule out the presence of an alteration. Some patients may require a biopsy for testing with FoundationOne CDx when archival tissue is not available which may pose a risk. When considering eligibility for ROZLYTREK® based on the detection of NTRK1/2/3 and ROS1 fusions, or for TEPMETKO® based on the detection of MET SNVs and indels that lead to MET exon 14 skipping, testing using plasma specimens is only appropriate for patients for whom tumor tissue is not available for testing. Patients who are tested with FoundationOne Liquid CDx and are negative for other companion diagnostic mutations should be reflexed to tumor tissue testing and mutation status confirmed using an FDA-approved tumor tissue test, if feasible. For the complete label, including companion diagnostic indications and important risk information, please visit www.F1CDxLabel.com and www.F1LCDxLabel.com.