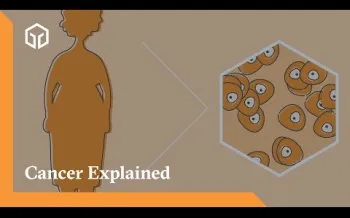
Cancer may occur when cells grow out of control and potentially spread into your body’s tissues. This happens because the DNA that tells a cell what to do is not working as it should. This is known as a alteration. Usually, our natural immune system can stop abnormal cell growth before problems happen. But when it doesn’t, cells keep growing and dividing.
In some cases, the cancer stays in one place (known as a localized tumor). In other cases, it spreads (known as a metastatic tumor). When tumors are metastatic, they consume the body’s resources as they grow and can damage healthy tissue and organs.