Our proven portfolio of tests and services brings in-depth genomic insights, experience, and a breadth of offerings into each practice we work with.
About Our Products and Services
Your Trusted Partner
Foundation Medicine has the most experience with comprehensive genomic profiling in the industry.* By partnering with biopharma and clinical stakeholders alike, we’ve developed an extensive genomic database and body of research that have strengthened the services we can offer all of our customers.
Our CGP Portfolio
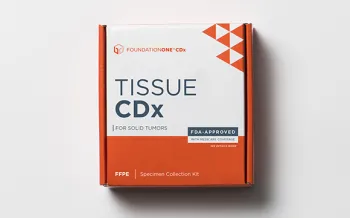
FoundationOne®CDx
FDA-approved tissue-based broad companion diagnostic for all solid tumors, indicated for 20+ targeted therapies.

FoundationOne®Liquid CDx
FDA-approved blood-based companion diagnostic for all solid tumors, indicated for 8 targeted therapies.

FoundationOne®Heme
A laboratory developed test for hematologic malignancies, sarcomas or solid tumors where known or novel gene fusion detection is desired.

We provide actionable insights into the genomics of both rare and common cancers, fueling discovery through commercialization by using a robust database and having deep experience with FDA approvals.
Foundation Facts & Figures
Over 1 Million
FOUNDATION MEDICINE REPORTS DELIVERED
800+
PEER-REVIEWED PUBLICATIONS
60%
OF ALL US CDX APPROVALS FOR NGS TESTING*
Recent Updates
Actionable Insights Upfront
We understand that treatment planning for your cancer patients can be complex. That’s why we’re updating our FoundationOne CDx and FoundationOne Liquid CDx reports to pull the “Professional Services” summary section to the first page. This update will bring information for all reported biomarker and genomic findings and our new “Report Highlights” section upfront, to help you focus on the critical findings for your patients.

FoundationOne®CDx Adds First Group-Based CDx Claim
The U.S. Food and Drug Administration (FDA) has approved FoundationOne CDx as a companion diagnostic for a group of BRAF inhibitors and BRAF/MEK inhibitors in combination for melanoma patients with BRAF V600E and BRAF V600E/K mutations. FoundationOne CDx is the first comprehensive genomic profiling test to receive approval for a group-based companion diagnostic (CDx) claim.1 Based on this approval, FoundationOne CDx is now approved as a CDx for current and future therapies that are approved under these group indications.2
Additional Notes
* Data on file, Foundation Medicine, Inc, 2021
1 U.S. Food & Drug Administration. List of Cleared or Approved Companion Diagnostic Devices. Content current as of December 9, 2021. Accessed December 9, 2021. www.fda.gov/medical-devices/vitro-diagnostics/list-cleared-or-approved-companion-diagnostic-devices-vitro-and-imaging-tools
2 FoundationOne CDx reports will include this indication by January 20th, 2022
Important Safety Information
FoundationOne®CDx and FoundationOne®Liquid CDx are qualitative next-generation sequencing based in vitro diagnostic tests for advanced cancer patients with solid tumors and are for prescription use only. FoundationOne CDx utilizes FFPE tissue and analyzes 324 genes as well as genomic signatures. FoundationOne Liquid CDx analyzes 324 genes utilizing circulating cell-free DNA and is FDA-approved to report short variants in 311 genes. The tests are companion diagnostics to identify patients who may benefit from treatment with specific therapies in accordance with the therapeutic product labeling. Additional genomic findings may be reported and are not prescriptive or conclusive for labeled use of any specific therapeutic product. Use of the tests does not guarantee a patient will be matched to a treatment. A negative result does not rule out the presence of an alteration. Some patients may require a biopsy for testing with FoundationOne CDx when archival tissue is not available which may pose a risk. When considering eligibility for ROZLYTREK® based on the detection of NTRK1/2/3 and ROS1 fusions, or for TEPMETKO® based on the detection of MET SNVs and indels that lead to MET exon 14 skipping, testing using plasma specimens is only appropriate for patients for whom tumor tissue is not available for testing. Patients who are tested with FoundationOne Liquid CDx and are negative for other companion diagnostic mutations should be reflexed to tumor tissue testing and mutation status confirmed using an FDA-approved tumor tissue test, if feasible. For the complete label, including companion diagnostic indications and important risk information, please visit www.F1CDxLabel.com and www.F1LCDxLabel.com.