Treating cancer means more than just dealing with the disease; it also means treating the whole person. Christine Bray, ovarian cancer survivor, shares her journey to remission after obtaining a Foundation Medicine comprehensive genomic profiling test.
Foundation Medicine is transforming cancer care by providing patients, physicians and researchers with a deep understanding of the genomic mutations that drive cancer.

For Providers
Foundation Medicine’s proven portfolio of tests and services offers the quality and commitment you need to help guide treatment strategies for your advanced cancer patients.

For Biopharma & Research Partners
We can support you from initial discovery to launch of a new therapy with our global portfolio of tests and services and our scientific and regulatory experts to fit your specific needs. As a leader in companion diagnostic (CDx) approvals, we are your ideal partner to help make your next breakthrough a standard of care.
Biopharma & Research Partner Resources

For Patients
Every cancer is unique. That’s why we are committed to providing specific genomic insights into your cancer to help your doctor guide a personalized care plan.
Patient Resources
Proven Leadership
Our unique knowledge base, FoundationCore®, is one of the world's largest cancer genomic databases. It is designed to evaluate the genomic landscape across cancer types to better understand tumor biology, molecular biomarkers, and which treatments might work for which patients. This all helps researchers and biopharma companies develop new therapies, design better clinical trials, and advance precision medicine.
Over 1 Million
FOUNDATION MEDICINE REPORTS DELIVERED
1,000+
PEER-REVIEWED PUBLICATIONS
>60%
OF ALL US CDX APPROVALS FOR NGS TESTING*
Valuable Insights, Actionable Options
Our tests help to identify the genomic alterations driving a patient's cancer and match them with relevant targeted therapies, immunotherapies, and clinical trial options. We also provide decision support services and technology solutions to help support patient care. Learn more about our products and services
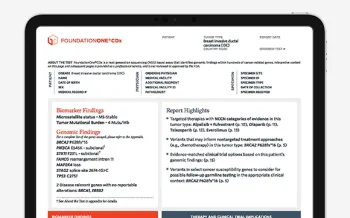
Actionable Insights Upfront
We understand that treatment planning for your cancer patients can be complex. That’s why we’re updating our FoundationOne CDx and FoundationOne Liquid CDx reports to pull the “Professional Services” summary section to the first page. This update will bring information for all reported biomarker and genomic findings and our new “Report Highlights” section upfront, to help you focus on the critical findings for your patients.
* Data on file, Foundation Medicine Inc., data as of Nov. 18, 2022.
Important Safety Information
FoundationOne®CDx and FoundationOne®Liquid CDx are qualitative next-generation sequencing based in vitro diagnostic tests for advanced cancer patients with solid tumors and are for prescription use only. FoundationOne CDx utilizes FFPE tissue and analyzes 324 genes as well as genomic signatures. FoundationOne Liquid CDx analyzes 324 genes utilizing circulating cell-free DNA and is FDA-approved to report short variants in 311 genes. The tests are companion diagnostics to identify patients who may benefit from treatment with specific therapies in accordance with the therapeutic product labeling. Additional genomic findings may be reported and are not prescriptive or conclusive for labeled use of any specific therapeutic product. Use of the tests does not guarantee a patient will be matched to a treatment. A negative result does not rule out the presence of an alteration. Some patients may require a biopsy for testing with FoundationOne CDx when archival tissue is not available which may pose a risk. When considering eligibility for ROZLYTREK® based on the detection of NTRK1/2/3 and ROS1 fusions, or for TEPMETKO® based on the detection of MET SNVs and indels that lead to MET exon 14 skipping, testing using plasma specimens is only appropriate for patients for whom tumor tissue is not available for testing. Patients who are tested with FoundationOne Liquid CDx and are negative for other companion diagnostic mutations should be reflexed to tumor tissue testing and mutation status confirmed using an FDA-approved tumor tissue test, if feasible. For the complete label, including companion diagnostic indications and important risk information, please visit www.F1CDxLabel.com and www.F1LCDxLabel.com.




