Driving Precision Medicine Forward in Cancer Care by Capitalizing on Actionable Insights
How comprehensive genomic profiling can open up new treatment opportunities, directly impacting patient care today.
Precision medicine is a reality today, connecting thousands of cancer patients to the latest treatment options. By personalizing every patient’s treatment strategy—guided and informed by genomic biomarker testing—precision medicine empowers healthcare providers to evaluate and predict which therapeutic approaches are most likely to be safe and effective in a specific person or group of people. In this way, precision medicine is the exact opposite of the conventional one-size-fits-all therapeutic approach, and is transforming the outcomes of patients with advanced cancer.

Comprehensive genomic profiling (CGP) is currently driving precision medicine in cancer care. With CGP, Foundation Medicine delivers actionable, tumor-specific and patient-specific insights that can enable dynamic decision-making for healthcare providers in the clinical setting. And each of these insights may help inform treatment strategies and open up new treatment opportunities.
What makes Comprehensive Genomic Profiling different?
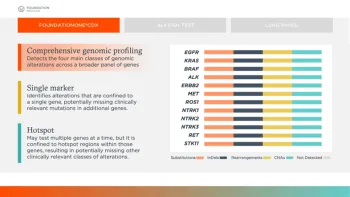
By its very definition, CGP is a marked advancement over single-gene or “hot spot” testing. Single-gene or “hot spot” testing identifies alterations that are confined to a single gene or commonly altered regions within those genes, resulting in potentially missing other clinically relevant mutations in additional genes or other clinically relevant classes of alterations. An incomplete picture of your patient’s cancer may mean missed opportunities for targeted treatment options.
In contrast to a single-gene or “hot spot” test, one CGP test can analyze hundreds of genes across the four main classes of alterations (substitutions, insertions/deletions, copy number alterations and rearrangements). This type of molecular testing can provide a broad and deep assessment of possible underlying oncogenic drivers that may or may not be targeted via traditional molecular testing methods. Furthermore, CGP testing can identify not only actionable genomic alterations, but also resistance gene mutations where targeted therapies may not have effect.
For example, the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Non-small Cell Lung Cancer (NSCLC)1 specifically recommend “broad molecular profiling” in eligible patients with metastatic NSCLC, which includes checking for alterations in EGFR, ALK, ROS1, BRAF, MET exon 14 skipping, RET, and NTRK1/2/3, and emerging biomarkers MET amplification and ERBB2 (HER2). * The NCCN Guidelines® also recommend testing for the immune biomarker PD-L1. Using sequential single biomarker or hotspot testing to cover all these recommended alterations and biomarkers carries the risk of exhausting time and tissue for patients.
*The NCCN Guidelines for NSCLC provide recommendations for certain individual biomarkers that should be tested and recommend testing techniques but do not endorse any specific commercially available biomarker assays or commercial laboratories.
Why comprehensive genomic profiling?
CGP tests can help healthcare providers and patients build a personalized treatment plan together that could avoid the pitfalls of a conventional trial-and-error approach when time is of the essence.
CGP tests can help:
- identify clinically relevant genomic alterations and associated targeted therapies for a patient’s specific cancer.
- identify the absence of pertinent genomic alterations that can help rule out therapies that may not be effective in treating a particular cancer, helping save time, resources, and impact on quality of life.
- match patients with appropriate ongoing clinical trials and experimental therapies.
What makes Foundation Medicine CGP different?
Only Foundation Medicine offers an FDA-approved portfolio of both tissue- and blood-based comprehensive genomic profiling tests for all solid tumors. Foundation Medicine has the most FDA-approved companion diagnostic claims on the market, across multiple cancer indications, which enhances the actionability of its reports. With over half a million patient samples profiled and over 500 peer-reviewed publications, Foundation Medicine has the most experience in CGP.2
Play your part in driving precision medicine forward
The adoption of CGP in the healthcare community has not been widespread.3 Yet, studies have shown that CGP will detect at least one actionable clinically relevant genomic alteration in most patients—up to 93.5% in one single center, prospective study with 339 patients who underwent CGP.4 Adopting CGP testing in your practice will help build personalized treatment plans for your cancer patients and uncover potential opportunities that conventional testing might miss.

As a leader in precision medicine, with 65+ biopharma partnerships, 500+ peer-reviewed publications and over half a million patient samples profiled,2 Foundation Medicine is revolutionizing cancer care. By providing insights and outcomes to optimize therapeutic decision-making, Foundation Medicine partners with healthcare providers to help transform the lives of advanced cancer patients.
References
1. Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for NSCLC V.5.2021. © National Comprehensive Cancer Network, Inc. 2020. All rights reserved. Accessed June 17, 2021. To view the most recent and complete version of the guideline, go online to NCCN.org. NCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way.
2. Data on file. Foundation Medicine, Inc. 2021.
3. Statz CM, Patterson SE, Mockus SM. Barriers preventing the adoption of comprehensive cancer genomic profiling in the clinic. Expert Rev Mol Diagn. 2017;17(6):549-555. doi:10.1080/14737159.2017.1319280
4. Wheler JJ, Janku F, Naing A, et al. Cancer Therapy Directed by Comprehensive Genomic Profiling: A Single Center Study. Cancer Res. 2016;76(13):3690-3701. doi:10.1158/0008-5472.CAN-15-3043
Questions? We’re Here to Help
Please contact our client services team by phone at +1(888) 988-3639 or by email at the link below.
Important Safety Information
FoundationOne CDx
FoundationOne®CDx is a qualitative next-generation sequencing based in vitro diagnostic test for cancer patients with solid tumors and is for prescription use only. The test analyzes 324 genes as well as genomic signatures including microsatellite instability (MSI) and tumor mutational burden (TMB) and is a companion diagnostic to identify patients who may benefit from treatment with specific therapies in accordance with the approved therapeutic product labeling. Additional genomic findings may be reported and are not prescriptive or conclusive for labeled use of any specific therapeutic product. Use of the test does not guarantee a patient will be matched to a treatment. A negative result does not rule out the presence of an alteration. Some patients may require a biopsy. For the complete label, including companion diagnostic indications and important risk information, please visit www.F1CDxLabel.com
FoundationOne Liquid CDX
FoundationOne®Liquid CDx is for prescription use only and is a qualitative next-generation sequencing based in vitro diagnostic test for cancer patients with solid tumors. The test analyzes 324 genes utilizing circulating cell-free DNA and is FDA-approved to report short variants in 311 genes and as a companion diagnostic to identify patients who may benefit from treatment with specific therapies (listed in Table 1 of the Intended Use) in accordance with the approved therapeutic product labeling. Additional genomic findings may be reported and are not prescriptive or conclusive for labeled use of any specific therapeutic product. Use of the test does not guarantee a patient will be matched to a treatment. A negative result does not rule out the presence of an alteration. When considering eligibility for certain therapies for which FoundationOne Liquid CDx is a companion diagnostic, testing of plasma is only appropriate where tumor tissue is not available. Patients who are negative for other companion diagnostic mutations should be reflexed to tumor tissue testing and mutation status confirmed using an FDA-approved tumor tissue test, if feasible. For the complete label, including companion diagnostic indications and complete risk information, please visit www.F1LCDxLabel.com.